

The amount of calcium carbonate (CaCO 3) incorporated in the cuttlebone is used as a measure here. The growth rate of the calcareous shield, the cuttlebone, also proceeded at very high rates (see the red and black bars in the diagram). The total weight of young animals increased over a period of 40 days in acidic seawater (red line) just as robustly as in water with a normal pH and CO 2 content (black line). In order to draw accurate conclusions about how the carbon dioxide increase in the water affects marine organisms, further studies are therefore necessary.Ģ.8 > Active and rapidly moving animals like the cephalopod mollusc (cuttlefish) Sepia officinalis are apparently less affected by acidification of the water. In contrast, for more active animal groups such as fish, salmon, and the cephalopod mollusc Sepia officinalis, no evidence could be found as to know that the carbon dioxide content in the seawater had an impact on growth rates. For many of these invertebrates not only carbonate production, but also the growth rate of the animal was affected.
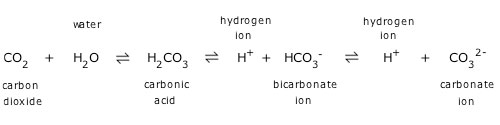
In other invertebrates species, such as mussels, sea urchins and starfish, a decrease in calcification rates due to CO 2 has also been observed. the organisms form more carbonate than is dissolved in the water), and thus the successful formation of reefs, will hardly be possible. Scientific studies suggest that carbon dioxide levels could be reached by the middle of this century at which a net growth (i.e. The best-known examples are the warm-water corals, whose skeletons are particularly threatened by the drop in pH values. Many marine organisms have already been studied to find out how acidification affects carbonate formation. Only when the animals are transferred to water with natural pH values do they start to build their protective skeletons again (c). Their small tentacles, which they use to grab nutrient particles in the water, are clearly visible. The animals take on an elongated polyp form. In acidic water (b) the carbonate skeleton degenerates. The animals live retracted within their carbonate exoskeleton (yellowish). Picture a shows a coral colony in its normal state. In extreme cases this can even lead to the dissolution of existing carbonate shells, skeletons and other structures.Ģ.7 > Studies of the coral Oculina patagonia show that organisms with carbonate shells react sensitively to acidification of the water. Report to the correct significant figures. Calculate the mass of precipitate that can be formed when starting with 25.48 mL of 0.067 M carbonic acid. Because carbonate ions together with calcium ions (als CaCO3) form the basic building blocks of carbonate skeletons and shells, this decline would have a direct effect on the ability of many marine organisms to produce biogenic carbonate. Science Chemistry Chemistry questions and answers Write the balanced chemical equation for the reaction between carbonic acid and iron (III) hydroxide. A 50 per cent decline of the levels is predicted, for example, if there is a drop in pH levels of 0.4 units.

(b) Write the net ionic equation for this. Over the long term, ocean acidification leads to a decrease in the concentration of carbonate ions in seawater. (a) Write a balanced molecular equation for the reaction of carbonic acid (H2CO3) and potassium hydroxide (KOH). This then reacts with carbonate ions and forms bicarbonate. In summary, the reaction of carbon dioxide in seawater proceeds as follows: First the carbon dioxide reacts with water to form carbonic acid. Calcium hydrogencarbonate is soluble in water. CO 2 + H 2O ↔ H 2CO 3 ↔ H + + HCO 3 – ↔ 2 H + + CO 3 2– The calcium carbonate precipitate reacts with more carbon dioxide to form calcium hydrogencarbonate, Ca(HCO3)2.
#Carbonic acid precipitate equation free#
This carbonic acid-carbonate equilibrium determines the amount of free protons in the seawater and thus the pH value. In particular, it is found that aluminum, magnesium and iron salts solubilized along with calcium by acid extraction of sludge precipitate as hydroxides at. carbon dioxide, carbonic acid, bicarbonate and carbonate ions, are referred to as dissolved inorganic carbon (DIC). All of the CO 2-derived chemical species in the water together, i.e. The carbonate reacts with CO 2 to form bicarbonate, which leads to a further uptake of CO 2 and a decline of the CO 3 2– concentration in the ocean. The reason for this is that bicarbonate and carbonate ions have been perpetually discharged into the sea over aeons. Seawater can assimilate much more CO 2 than fresh water. When carbon dioxide mixes with the water it is partially converted into carbonic acid, hydrogen ions (H +), bicarbonate (HCO 3 –), and carbonate ions (CO 3 2–). In the dissolution process, carbon dioxide reacts with the water molecules according to the equation below.

This is well known in mineral water, which often has carbon dioxide added. The atmospheric gas carbon dioxide (CO 2) dissolves very easily in water. \).When carbonate formation loses equilibrium


 0 kommentar(er)
0 kommentar(er)
